A double displacement reaction is also called a double replacement reaction salt metathesis reaction or double decomposition. H OH- H 2 O.

Chemical Reactions 1 Of 11 Double Replacement Reactions An Explanation Youtube
There are 5 types of double displacement reaction as shown below.

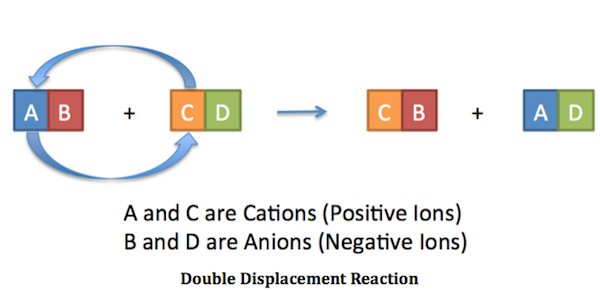
. The ions in compounds AB and CD switch partners. You will combine two water solutions each containing positive and negative ions. DOUBLE REPLACEMENT REACTIONS.
Because the replacement occurs in two places it is referred to as a double replacement. The driving force behind double-replacement reactions is the formation of a stable product. This type of reaction will take place in aqueous solution when one of the.
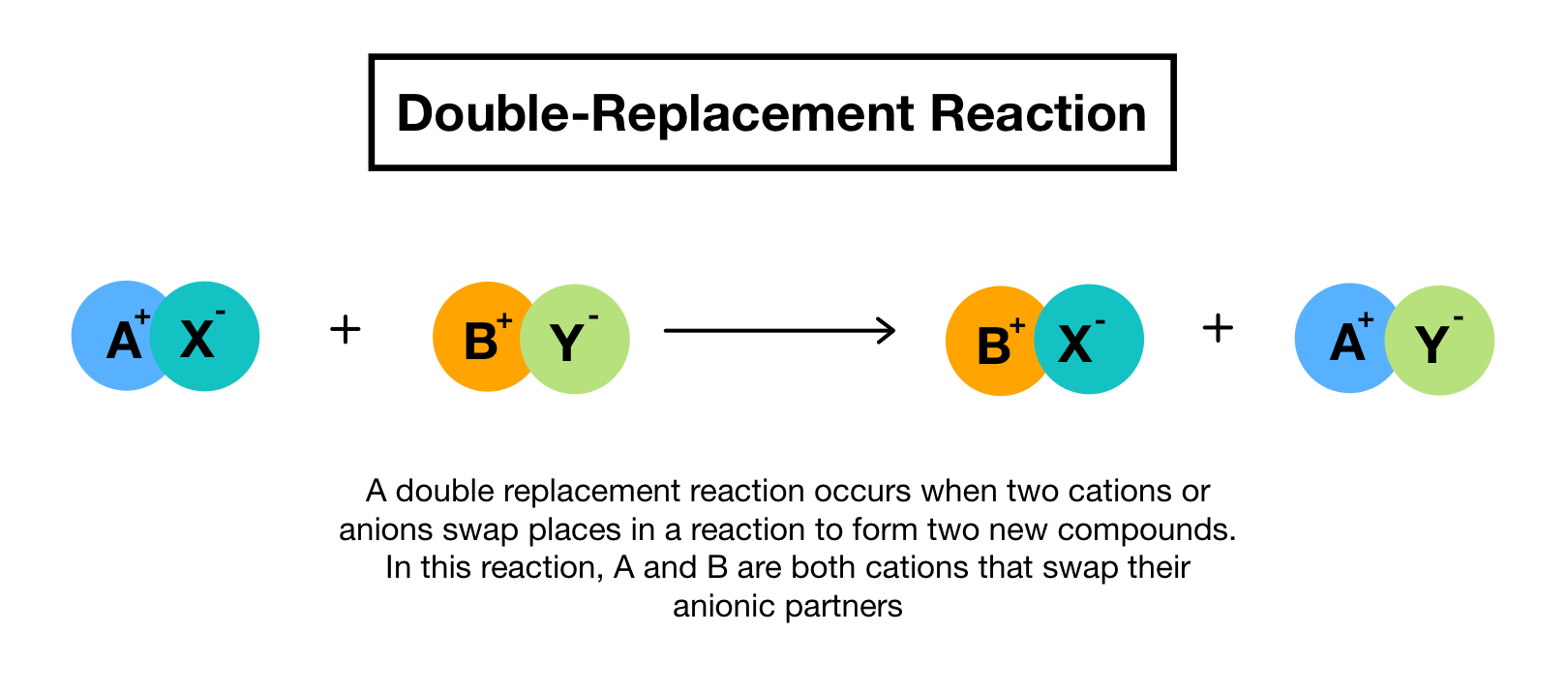
A double-replacement reaction exchanges the cations or the anions of two ionic compounds. The name describes the process very well. In a double displacement reaction atoms from two different compounds switch places.
Solubility rules are used to predict whether some double-replacement reactions will occur. In a double replacement reaction two compounds swap atoms forming two different compounds. Chemistry Double Replacement Reaction Practice Reactions Key Energy and the Human Journey Where We Have Been Where We.
You can think of the reaction as swapping the cations or the anions but not swapping both since you would end up with the same substances you started with. A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products. Double replacement reactions have the form.
Double displacement reactions occur when two ionic compounds react or when an ionic compound reacts with an acidu000bu000b It only occurs in liquids. Reaction to Keratin Hair Treatment Short Hair Styles. This chemistry video tutorial explains how to identify the products of a double replacement reaction from a sentence or word problem.
There is one other way a double-replacement reaction can happen. Fe2O3 6HCl 2FeCl3 3H2O. A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate.
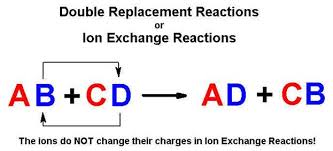
Double displacement reactions can be further classified as. Two replacements take place in. AB CD AD CB.
The reaction occurs most often between ionic compounds although technically the bonds formed between the chemical species may be either ionic or covalent in nature. Solubility rules are used to predict whether some double-replacement reactions will occur. Describes the basics of double replacement reactions how to identify them predict the products and balance the chemical equation.
The name describes the process very well. A double replacement reaction is a type of chemical reaction. However heat is given off and the pH of both liquids move closer to a.
Video on recognizing and understanding double replacement reactions. Double replacement reactions are also called metathesis or double displacement reactions. You will study double displacement reactions using a small-scale method and predict the products of double displacement reactions.
Double replacement sometimes referred to as double displacement reactions are when parts of ionic compounds are switched to form two new ionic compounds. Two examples are also sho. Acid carbonate salt H2O CO2u000b.
Return to Autism Page Heartfixer. Typically you will be given the left-hand reactant side and asked to provide the products to the reaction. A precipitation reaction is usually a double displacement reaction.
Consider this generalized reaction between two ionic compounds. Usually in these reactions when combining. They are included in the single replacement category.
Its when an acid H ions neutralizes hydroxide ions OH- to form water H 2 O. There are no precipitate or bubbles to show this occurred. Diatomic elements do not count.
Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. The reactants are two compounds and the products are two different compounds. Beckman Coulter UniCel DxC Synchron 800 Instructions For.
In double replacement both reactants are compounds each with a cation part and an anion part. A double-replacement reaction exchanges the cations or the anions of two ionic compounds. Gas Ionic molecular compound 3 more precipitate double-displacement reaction double-replacement reaction.
The way I think of it since were dealing with ionic compounds is that when I write out a reaction I. A double-replacement reaction sometimes referred to as a double-displacement reaction occurs when parts of two ionic compounds are exchanged making two new compounds. It covers three types.
Thats a sign that the double-replacement reaction is occurring. A precipitation reaction is a double-replacement reaction in which one product is a solid precipitate. The ions that do not form the precipitate usually form the solution.
SCICHE515 Double-Replacement Reactions - Chemistry. The solvent for a double replacement reaction is usually water and the reactants and products are usually ionic compoundsbut they can also be acids or bases. A double replacement reaction is represented by the general equation.
Salt salt insoluble salt saltu000b. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.

Double Replacement Double Displacement Reaction
Double Replacement Reactions Definition Examples Expii

Double Replacement Reaction Definition And Examples
What Is A Double Replacement Reaction In Chemistry Socratic

Single Replacement Reaction Definition And Examples
Double Replacement Reactions Chemistry Socratic

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

0 comments
Post a Comment